Abstract
Introduction: Rituximab is a chimeric mouse/human monoclonal antibody that binds to CD20, which is expressed on both normal and neoplastic B-lymphocytes. The addition of rituximab to standard chemotherapy regimens has led to improvements in progression-free survival and overall survival for diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin lymphoma (NHL). Despite the widespread clinical success of rituximab, its mechanism of action is not clear. However, evidence from pre-clinical models and clinical observations suggest complement-mediated cytotoxicity (CMC) and/or antibody-dependent cell mediated cytotoxicity (ADCC) may both play a role.
Clinically, the most common complication of rituximab is mild infusion reactions (IR), characterized by fevers, rigors, flushing, dyspnea, and mild hypotension, which are typically limited to the first infusion. Complement activation has been suggested as an underlying mechanism for rituximab-related IR, though the exact nature or implications of such reactions are not known. Since complement activation is also a potential mechanism of rituximab-related anti-lymphoma activity, we aimed to determine whether an association exists between rituximab associated IR and outcomes in patients with DLBCL. These results would help guide future studies on the mechanism of monoclonal antibody therapy for NHL.
Methods: We identified and analyzed a retrospective cohort of 229 patients with DLBCL. They were stratified into two cohorts, based on the presence or absence of an infusion reaction during the first cycle of rituximab. The Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 was used to grade the severity of rituximab-associated IR. Univariate and multivariate analyses were performed to evaluate the prognostic significance of rituximab-related infusion reactions relative to DLBCL subtype, IPI score, c-Myc mutational status, chemotherapy regimen, and Ki-67 proliferative index.
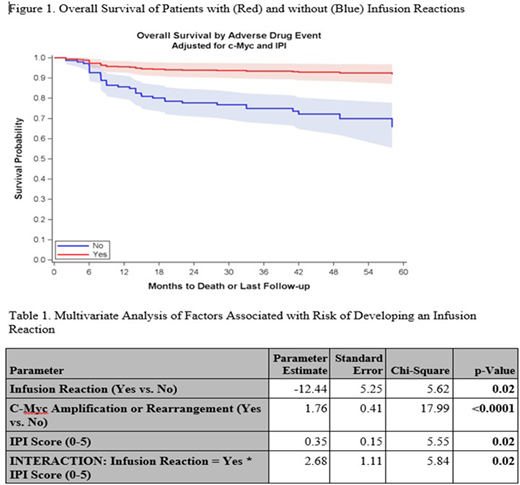
Results: Baseline characteristics did not differ significantly between the two groups. Rituximab was included as initial treatment in all patients studied. Among 226 patients, 68 (30.1%) had a grade 1-2 IR, all of whom were able to receive subsequent rituximab therapy. In comparing outcomes between the two cohorts, those with an IR had a significantly greater overall survival (HR 0.26, 95% CI 0.07-0.95) at five years (Figure 1). Multivariate analysis showed that patients with c-Myc alterations or higher IPI scores were more likely to experience a rituximab associated IR (Table 1). Furthermore, subgroup analysis showed that among patients with either c-Myc alterations or GCB subtype of DLBCL, those who experienced an IR had a statistically significant increase in progression-free survival (p-value of 0.01 and 0.03, respectively).
Conclusion: Rituximab associated IR occurs commonly and is associated with better overall survival in patients with DLBCL. Subgroup analyses suggest that this benefit is largely restricted to patients with c-Myc alterations or the GCB subtype. While these data are limited by the small sample size and retrospective nature, they do generate some intriguing hypotheses related to underlying mechanism of action of rituximab in a subset of DLBCL patients. These data have potential implications in augmenting the efficacy of CD20-directed monoclonal antibody therapy in these patients that should be further validated in subsequent prospective studies.
Bartlett:Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding; Astra Zeneca: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Meyers Squibb: Research Funding; Novartis: Research Funding; Affimed: Research Funding; Forty Seven: Research Funding; ImaginAB: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Immune Design: Research Funding; Millennium: Research Funding; Merck & Co: Research Funding. Wagner-Johnston:JUNO: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Novartis: Research Funding; Celgene: Research Funding; ASTEX: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal